Introduction
Chronic Lymphocytic Leukemia (CLL) is a mature neoplasm characterized by the proliferation of immunologically dysfunctional B cells. Inhibitors of BCL2 (venetoclax) and Bruton Tyrosine Kinase (BTK) (ibrutinib, acalabrutinib and zanubrutinib) are novel agents that have revolutionized outcomes in CLL patients in the newly diagnosed and relapsed/refractory settings. Nevertheless, tumor lysis syndrome (TLS) and gastrointestinal bleeding (GIB) remains concerning adverse events. In clinical trials, fasting has demonstrated reductions in chemotherapy-related side effects and improved treatment tolerability . However, with novel agents in CLL this can be challenging with the need for aggressive hydration in fluid-restricting fasting practices and the higher risk for GI bleeding and peptic ulcer disease complications with fasting. This review aims to explore the literature available to answer the permissibility of intermittent fasting (IF) in CLL patients who are being treated with first line novel agents (FLNAs).
Methods
Literature exploration was performed to identify IF practices and the effects of fasting conditions and fluid-restriction on the pharmacokinetics (PK) of FLNAs. Literature was also scoped for data on TLS and GIB risk associated with FLNAs in CLL patients as well as the effects of fasting and or fluid-restricted states on GIB and TLS risks. Lastly, the identified risks were accumulated to build a pathway for permissibility of IF in this patient population.
Results
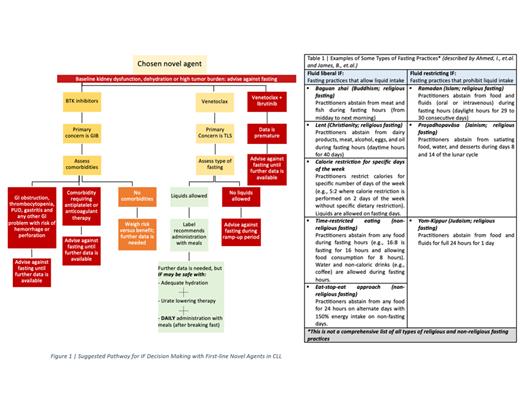
Venetoclax has amplified the historical low risk of TLS in patients with CLL to a significant degree. Approaches to prevent TLS such as aggressive hydration and use of uric acid reducing drugs hinder patients wishing to practice fluid restricting IF ( See Table 1 and Figure 1), especially during ramp-up phase. Moreover, venetoclax should be administered with food and on daily basis. Hence, only fluid liberal IF practices with daily food intake and medication administration may be possible in venetoclax CLL patients.
Furthermore, studies have indicated a possible increased baseline risk of GIB in patients who practice IF. Also, FDA reporting system for adverse events (AE) had higher GIB events among ibrutinib patients compared to other FLNAs. Overall, there was scarcity in the clinical data available on the effects of IF on treatment outcomes with FLNA in CLL patients. There was also evident variability in the number of reported AE between the relatively older (ibrutinib and venetoclax) and newer medications (acalabrutinib and zanubrutinib), which may be causing an underestimation of treatment risks.
Conclusion
Until further data is available, patients on BTK inhibitors should refrain from practicing IF for GI bleeding risk. However, it may be possible for patients on venetoclax to practice fluid liberal IF conditionally and conservatively for TLS risk. This is on the conditions that adequate hydration and daily administration with food are achieved.
Fasting's risks and benefits must be discussed with patients . Further prospective clinical trials, including exploration of different forms of IF, are needed to better elucidate the effect of IF on FLNAs treatment -related outcomes in CLL patients .
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal